
Get Back to Living Your Life
At Stem Cell Rejuvenation Institute, we provide non-surgical solutions and
minimally invasive options to help you get back to doing the things you love.
Get Back to Living Your Life
At Stem Cell Rejuvenation Institute, we provide non-surgical solutions and
minimally invasive options to help you get back to doing the things you love.
Stem Cell Rejuvenation Institute

Specialists focused on regenerative medicine through a set of innovative processes that use biological material (cells and tissues) aimed at repairing a tissue or the function of an organ through the stimulation and induction of its own self-regeneration to improve and prevent certain conditions or diseases through the use of mesenchymal stem cells and their protein extracts (exosomes and growth factors).
Stem Cell Rejuvenation Institute
We specialize in regenerative medicine, utilizing advanced techniques that harness biological materials—like cells and tissues—to restore tissue and organ functions. By tapping into the body's natural self-healing abilities, we target and enhance self-regeneration. Our approach centers on the powerful potential of mesenchymal stem cells, complemented by protein extracts such as exosomes and growth factors, to combat and prevent various conditions and diseases.

Our Process
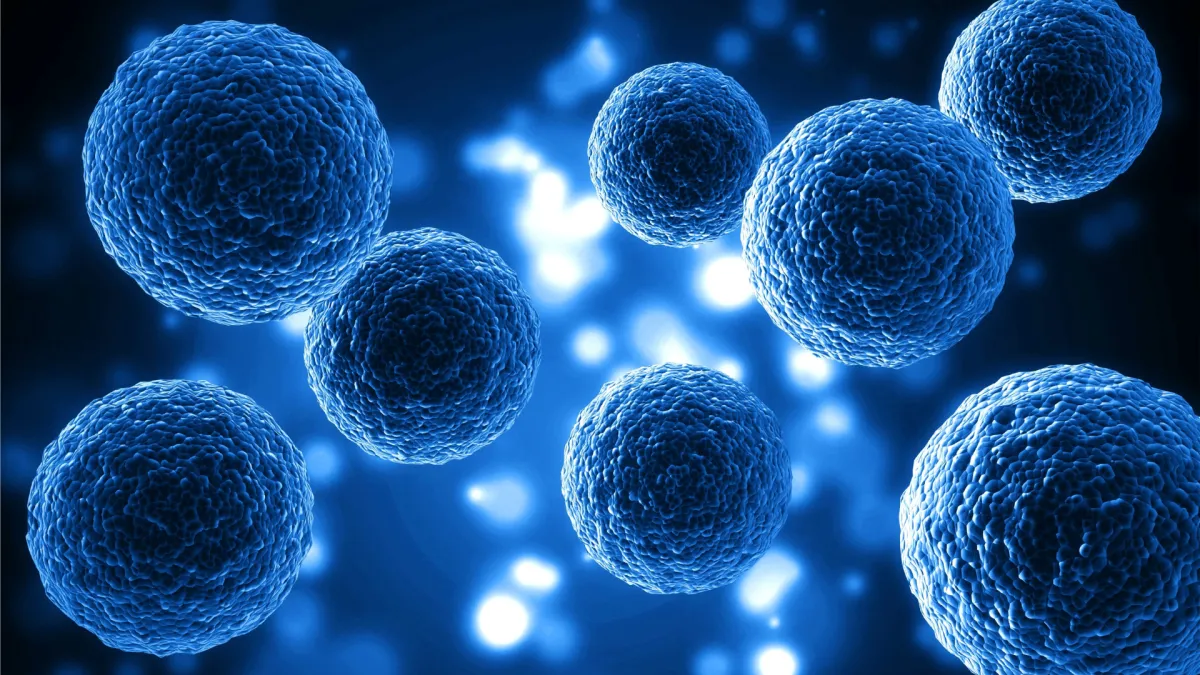
CELLULAR THERAPY WITH MESENCHYMAL STEM CELLS
Mesenchymal stem cells (MSCs) can differentiate into various types of cells in skeletal tissues, such as cartilage, bone, and fat. Mesenchymal Stem Cells are multipotent adult stem cells that can differentiate into various cell types maintaining a high capacity for self-renewal, helping to regenerate damaged tissues and organs, improve their function, modulate the immune system and reduce inflammation, allowing a better quality of life.

Exosomes and Secreted Growth Factors by MSC
EXOSOMES:
Extracellular vesicles secreted by mesenchymal stem cells. They allow intercellular communication; inside are proteins, lipids, and other important nutritional factors for cells. They are between 30 and 150 nanometers in size. Due to their small size, they have a great capacity to be entered by the cells of the dermis and epidermis.
GROWTH FACTORS SECRETED BY MESENCHYMAL STEM CELLS:
Our Process
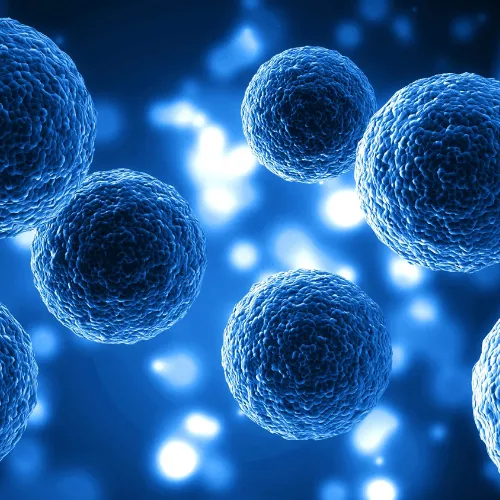
Cellular Therapy With Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) can differentiate into various types of cells in skeletal tissues, such as cartilage, bone, and fat. Mesenchymal Stem Cells are multipotent adult stem cells that can differentiate into various cell types maintaining a high capacity for self-renewal, helping to regenerate damaged tissues and organs, improve their function, modulate the immune system and reduce inflammation, allowing a better quality of life.

Exosomes and Secreted Growth Factors by MSC
EXOSOMES: Extracellular vesicles secreted by mesenchymal stem cells. They allow intercellular communication; inside are proteins, lipids, and other important nutritional factors for cells. They are between 30 and 150 nanometers in size. Due to their small size, they have a great capacity to be entered by the cells of the dermis and epidermis.
GROWTH FACTORS SECRETED BY MESENCHYMAL STEM CELLS:
Book An Appointment Now
You have the power to take control of your life. Seize the opportunity to make a difference and take the first step towards a transformative journey.
Book An Appointment Now
You have the power to take control of your life. Seize the opportunity to make a difference and take the first step towards a transformative journey.
Regenerative Medicine
Unlock the power of regenerative medicine, where we harness the potential of human and animal cells to rejuvenate, reconstruct, and revitalize. Step into a future where we don't just treat; we transform to restore optimal health and vitality.
Stem Cell Treatments
Cartilage Regeneration
Platelet-Rich Plasma (PRP)
Prolotherapy
Regenerative Medicine
Unlock the power of regenerative medicine, where we harness the potential of human and animal cells to rejuvenate, reconstruct, and revitalize. Step into a future where we don't just treat; we transform to restore optimal health and vitality.
Stem Cell Treatments
Cartilage Regeneration
Platelet-Rich Plasma (PRP)
Prolotherapy

BEST PROFESSIONALS!

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Lorem odio diam, at sociis pharetra, orci volutpat est viverra. Viverra pharetra, pretium, a tellus imperdiet. Nisl non elementum tincidunt mollis neque. Et sagittis purus tincidunt tincidunt
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Lorem odio diam, at sociis pharetra, orci volutpat est viverra. Viverra pharetra, pretium, a tellus imperdiet. Nisl non elementum tincidunt mollis neque. Et sagittis purus tincidunt tincidunt
The team at Stem Cell Rejuvenation Institute
Thousands of patients have been expertly guided by our team toward a healthier life.

Dr. Vincent J. Bennett
Dr. Bennett is a board-certified physician with 15+ years' experience in Los Angeles. He studied at Temple, Georgetown, and Howard Universities before his residency in LA. Notably, he's been a medical director at St. Vincent's and Hollywood Presbyterian Hospitals.
Dr. Bennett's expertise extends to stem cells, with lectures, consultations, and leading clinical investigators. His focus is on improving clients' quality of life through evidence-based stem cell applications.

Janae Brand
Janae Brand, President of SkinSultant Management Services, is a respected innovator in medical aesthetics and anti-aging with a successful track record spanning 25 years. Her expertise lies in skincare, beauty treatments, and non-ablative lasers. She is also certified in Integrative Nutrition, crafting customized plans for health and weight management.
Janae's philosophy revolves around promoting only what she personally believes in and has experienced success with. She partners with The Stem Cell Rejuvenation Institute in Mexico to offer Mesenchymal Stem Cell treatments.
What client say about us

Lorem Ipsum is simply dummy text of the printing
and typesetting industry. Lorem Ipsum has been
he industry's standard dummy text ever since the
1500s, when an unknown printer took a galley of
type and scrambled.

Darcel Ballentine
Barone LLC.

Lorem Ipsum is simply dummy text of the printing
and typesetting industry. Lorem Ipsum has been
he industry's standard dummy text ever since the
1500s, when an unknown printer took a galley of
type and scrambled.

Darcel Ballentine
Barone LLC.
What client say about us

Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled.

Darcel Ballentine
Barone LLC.

Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled.

Leatrice Handler
Acme Co.
Frequently Asked Question
What makes our cells better?
We manufacture the cells in a controlled environment that is optimized for generating a final product with high anti-inflammatory properties. During the manufacturing process we supercharge the stem cells by training them to survive harsh environments often found in diseased or damaged tissues. CPI doesn’t use any type of stem cell; we have highly specialized stem cells that have increased therapeutic properties over other stem cells.
Using our in-house manufacturing facility, we are able to generate billions of cells that are highly therapeutic and targeted to areas suffering from an acute or chronic inflammatory condition.
Why can't I get these same stem cell treatment in the USA?
Current stem cell treatments in the United States are being performed under FDA approved clinical trials, and although the data is very encouraging, final FDA approval for many of these treatments is still years away. SCRI is regulated by national regulatory agencies, COFEPRIS or the Federal Committee for Protection from Sanitary Risks (Comisión Federal para la Protección contra Riesgos Sanitarios) is the equivalent of the FDA in Mexico. SCRI is approved by COFEPRIS to perform cell therapy that in many regions such as the United States is years away from being a treatment option.
Is there documented medical proof that this works?
SCRI uses protocols published in peer-reviewed medical journals that have demonstrated the therapeutic potential of cell-based intervention. Depending on the geographical location, some of these therapies may be approved or in clinical trials. We always do our best, but nothing works for everyone.
How long will this last?
Every patient responds to therapy differently; some have very fast therapeutic benefits, while others have a more gradual long-term improvement.
Do you have an aftercare program?
Our aftercare team will continue to monitor your healing with follow-up calls. They are also available to review any exams or MRIs post-treatment with you.
Do I have to be on a special diet to do stem cells?
The Healthier, the better: organic, low sugars, low inflammatory, No Alcohol for as long as possible before and after treatment.
What are the contraindications for getting stem cells? Can anyone be a patient?
Example, a patient with a healthy medical history with multiple injuries probably will not have any contraindications to being treated.
Where do stem cells come from?
Our stem cells are isolated from umbilical cord, which is a rich source of many different cell types, including a stem cell population that SCRI has isolated. We manufacture the cells in a controlled environment that is optimized for generating a final product with high anti-inflammatory properties. Using our in-house manufacturing facility, we are able to generate billions of cells that are highly therapeutic and targeted to areas suffering from an acute or chronic inflammatory condition.
Do I need more than one treatment?
Every patient is unique and responds differently. Some patients are quick responders and experience sustained results after a single treatment, others respond gradually over time and may require additional treatments in the future to continue improving their clinical outcome. Our doctors will work with you to develop a treatment strategy for optimal results.
How do I know if I need Stem Cells?
To determine if you qualify for stem cell therapy, our doctors will need to review your medical. Please contact our patient care team at 1 (833) 274-4442 (toll-free in the U.S.). We are happy to answer any and all of your questions.

Book An Appointment Now
Take the first step towards the healthier life you deserve.

Our medicine is founded on clinical use of stem cells.
Quick Links
About Us
Services
Contact Us
Social Media Links

©2023 – Stem Cell Rejuvenation | All Right Reserved
